Synthetic Fluorination Methodology
Fluorination of a drug candidate affects physicochemical properties, which in medicinal settings perturbs pharmacodynamic, pharmacokinetic, distribution, and/or metabolic profiles both in vitro and in vivo. Thus, the ability to selectively install fluorinated groups under mild conditions is essential for accessing new therapeutics and biological probes. However, the unique physical properties of fluorinated substrates and/or reagents typically perturb standard organic reactivities, which impedes the extrapolation of many standard transformations to fluorinated systems. Thus, innovative catalyst systems, reagents, and synthetic strategies are required to generate target substructures. Additionally, the unique properties of fluorinated substrates enable new reactivities that cannot be achieved by the respective non-fluorinated counterparts, which provides opportunities to develop innovative reactions that exploit non-classical fluorinated activating groups provides to develop complementary and innovative transformations for accessing medicinally relevant substructures.
Decarboxylative coupling represents a powerful method for the construction of C–C bonds, and has gained special appreciation in non-fluorous chemistry. This strategy exhibits several appealing features, including: 1) the use of inexpensive and readily accessible starting materials; 2) the ability to selectively generate and couple reactive species under mild reaction conditions; 3) the release of CO₂ as a benign and easily removed by-product. Because of these benefits, we use decarboxylative strategies for developing new methods that streamline the synthesis of target molecules, and provide access to new fluorinated substructures that are challenging to access otherwise. In this area, the group collaborates with the Cheong Group at Oregon State University whose expertise in computational chemistry provides mechanistic insights into unusual reactivities.
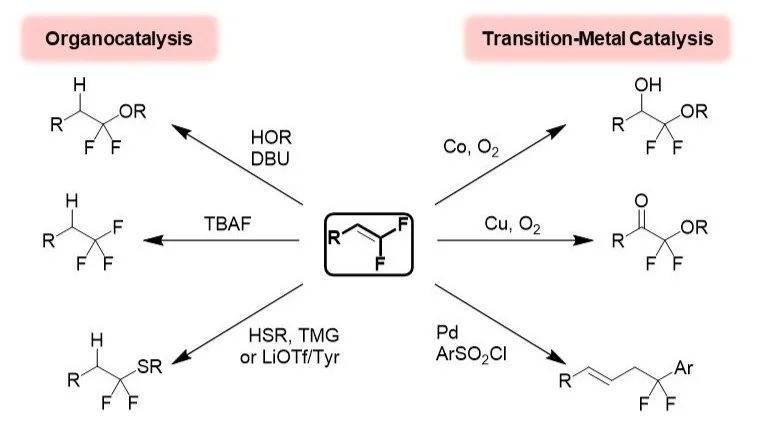
Alkenes serve as useful building blocks for many synthetic transformations, and in recent years, considerable attention has focused on both the syntheses and functionalization of these substructures. In this area, we develop strategies for preparing fluorinated and fluoroalkylated alkenes and functionalizing these substructures. In the latter case, the unique physicochemical perturbations imparted by the fluorine substituents enable catalytic regioselective functionalization reactions that are distinct from reactions of the nonfluorinated alkene counterparts.