29. Synthesis of Leu-Enkephalin Peptidomimetics Containing Trifluromethylalkenes as Amide Isopolar Mimics
Eeda, V.; Selvaraju, M.; Altman, R. A.*
J. Fluorine Chem. 2019, 218, 90–98.
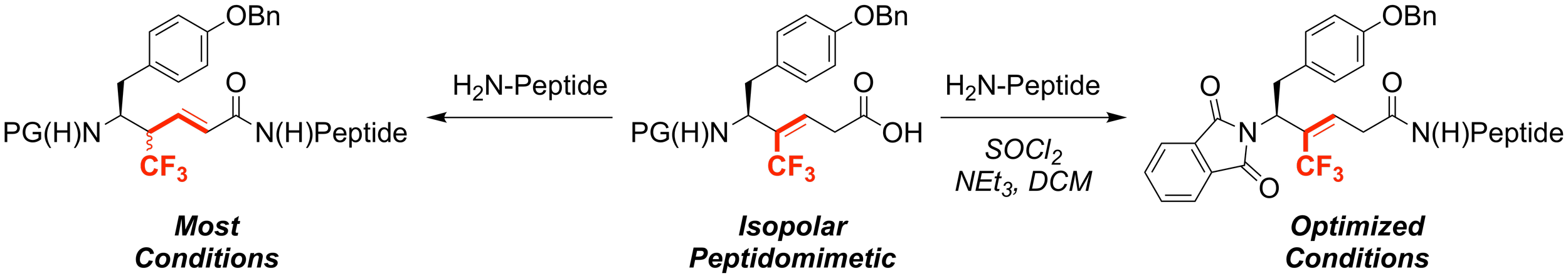
Fluorinated peptidomimetics are valuable substrates for exploring peptide backbone conformations and for perturbing physicochemical properties of probe compounds. However, in some cases synthetic limitations restrict installation of the fluorinated peptidomimetics into the desired probe compounds. For instance, trifluoromethylalkenes have served as amide isopolar mimics, but are rarely utilized, because many standard peptide-coupling conditions promote the isomerization of the alkene to thermodynamically favored positions. To address this challenge, we report the conversion of a naturally occurring amino acid to a Tyr1-ψ[CF3C = CH]-Gly2 dipeptide mimetic, and notably, successful peptide coupling reactions that avoid alkene isomerization. Using this strategy, we generated trifluoromethylalkene-containing Leu-enkephalin peptidomimetics in high purity and good yield. This sequence suggests that the trifluoromethylalkene peptidomimetics can be incorporated into other target molecules with appropriate optimization.