
34. The Meta-Position of Phe4 in Leu-enkephalin Regulates Potency, Selectivity, Functional Activity, and Signaling Bias at the Delta and Mu Opioid Receptors
Cassell, R. J.; Sharma K. S.; Su, H.; Cummins, B. R.; Cui, H.; Mores, K. L.; Blaine, A. T.; Altman, R. A.;* van Rijn, R. M.*
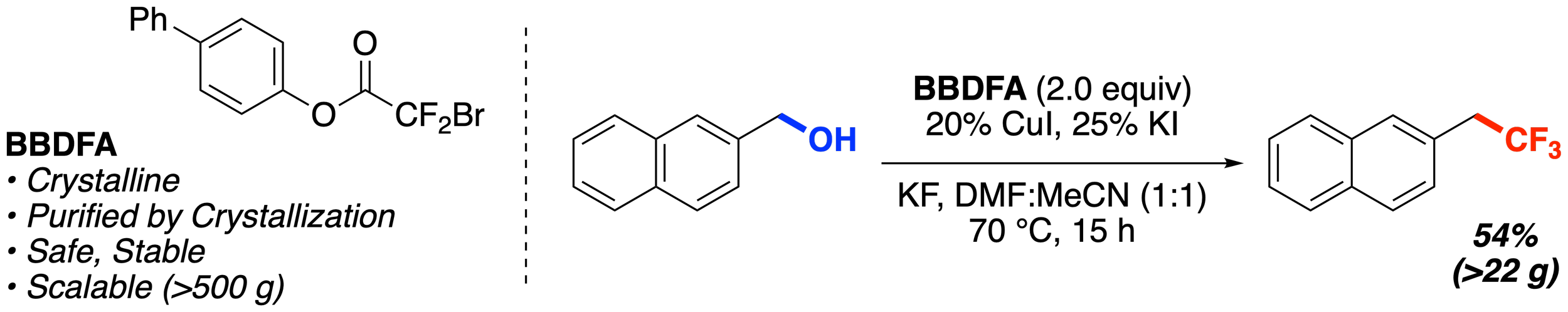
33. BBDFA: A Practical Reagent for Trifluoromethylation of Allylic and Benzylic Alcohols on Preparative Scale
Han, C.;* Alabanza, L. M.; Kelly, S. M.; Orsi, D. L.; Gosselin, F.; Altman, R. A.*

32. Organocatalytic Strategy for Hydrophenolation of gem-Difluoroalkenes
Orsi, D. L.; Yadav, M. R.; Altman, R. A.*
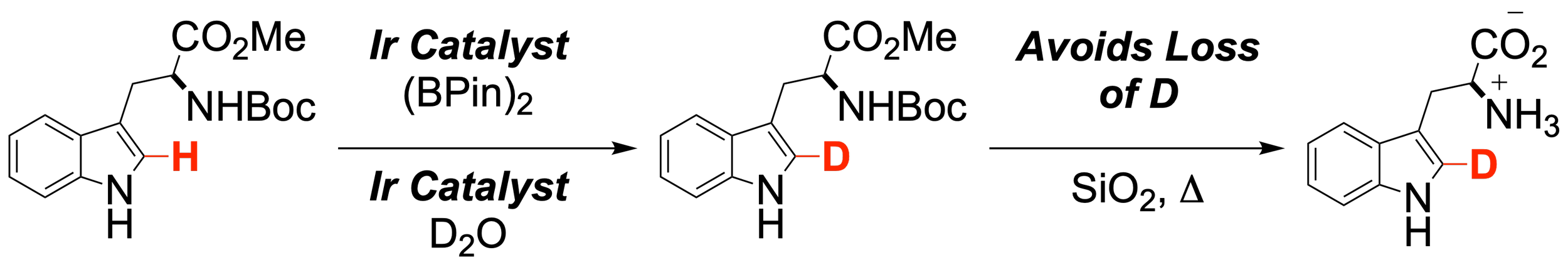
31. Synthesis of C2-Deutero-L-Tryptophan by Sequential Ir-Catalyzed Reactions
Vallakati, R. K.; Plotnikov, A. T.; Altman, R. A.*
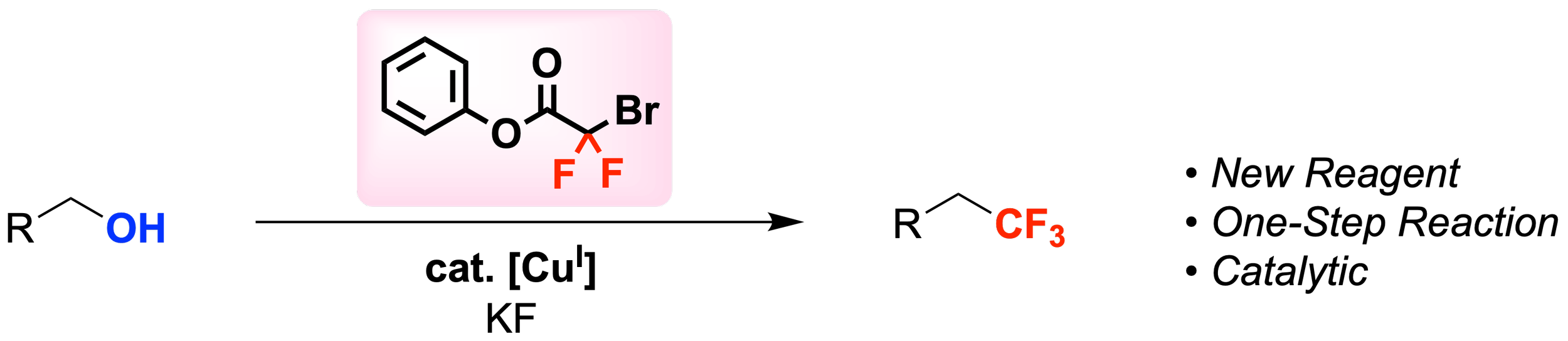
30. Catalytic One-Step Deoxytrifluoromethylation of Alcohols
de Azambuja, F.; Lovrien, S. K.; Ross, P.; Ambler, B. R.; Altman, R. A.*
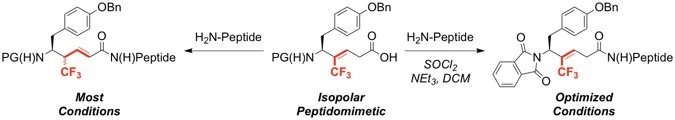
29. Synthesis of Leu-Enkephalin Peptidomimetics Containing Trifluromethylalkenes as Amide Isopolar Mimics
Eeda, V.; Selvaraju, M.; Altman, R. A.*
![28. Tyr1-ψ[(Z)CF═CH]-Gly2 Fluorinated Peptidomimetic Improves Distribution and Metabolism Properties of Leu-Enkephalin](https://images.squarespace-cdn.com/content/v1/615f42729a3e7155f1428c2e/1757716670708-LLAKP8S2PMCIACEWBGBF/28+ACS+Chem+Neurosci+FPM+DMPK.png)
28. Tyr1-ψ[(Z)CF═CH]-Gly2 Fluorinated Peptidomimetic Improves Distribution and Metabolism Properties of Leu-Enkephalin
Altman, R. A.*; Sharma, K. K.; Rajewski, L. G.; Toren, P. C.; Baltezor, M. J.; Pal, M.; Karad, S. N.
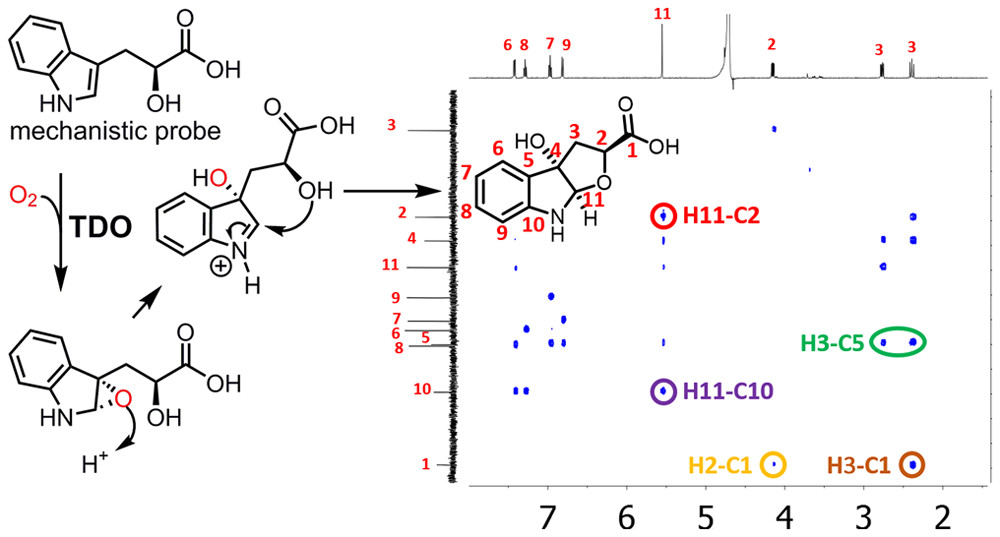
27. Stepwise O-Atom Transfer in Heme-based Tryptophan Dioxygenase: The Role of Substrate Ammonium in the Epoxide Ring Opening
Shin, I.; Ambler, B. R.; Wherritt, D.; Griffith, W.; Maldonado, A.; Altman, R. A.;* Liu, A.*
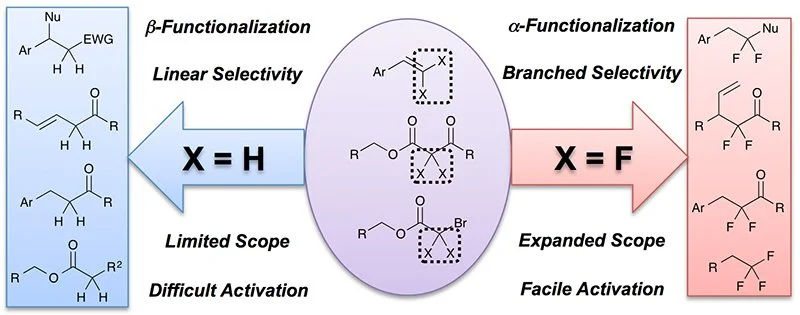
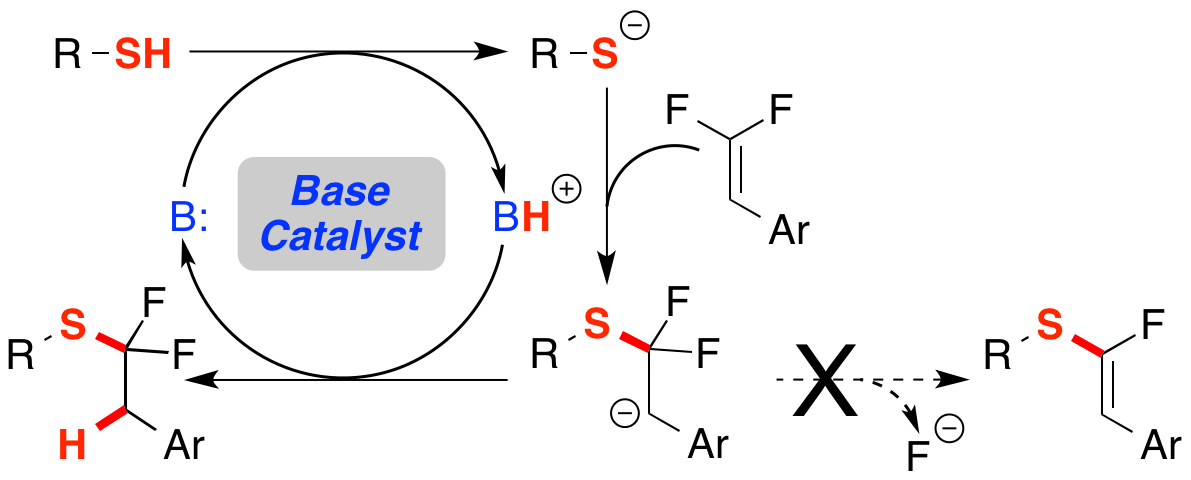
25. Base Catalysis Enables Access to α,α-Difluoroalkylthio-ethers
Orsi, D. L.; Easley, B. J.; Lick, A. M.; Altman, R. A.*
![24. Synthesis and Opioid Activity of Tyr1–ψ[(Z)CF=CH]–Gly2 and Tyr1–ψ[(S)/(R)-CF3CH–NH]–Gly2 Leu-enkephalin Fluorinated Peptidomimetics](https://images.squarespace-cdn.com/content/v1/615f42729a3e7155f1428c2e/1757716737403-7N9EEC7VX8ZCKO12X9HG/24+ChemMedChem+Enkephalin+FPMs.png)
24. Synthesis and Opioid Activity of Tyr1–ψ[(Z)CF=CH]–Gly2 and Tyr1–ψ[(S)/(R)-CF3CH–NH]–Gly2 Leu-enkephalin Fluorinated Peptidomimetics
Karad, S. N.; Pal, M.; Crowley, R. S.; Prisinzano, T. E.; Altman, R. A.*
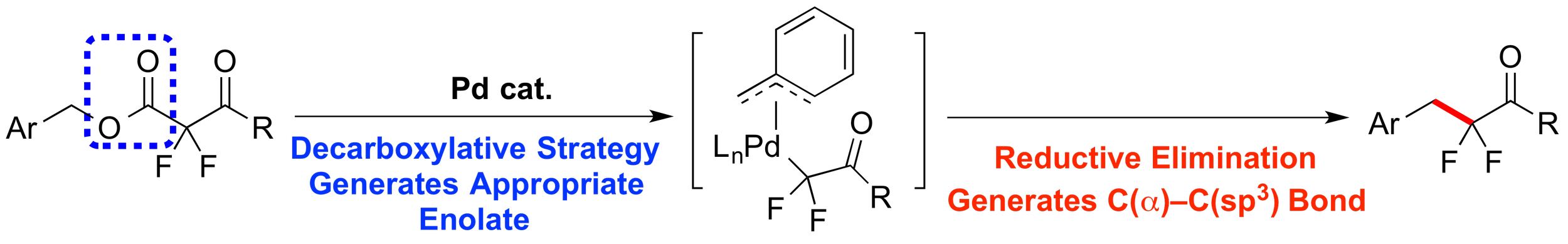
22. Palladium Catalysis Enables Benzylation of α,α-Difluoroketone Enolates
Yang, M.-H.; Hunt, J. R.; Sharifi, N.; Altman, R. A.*
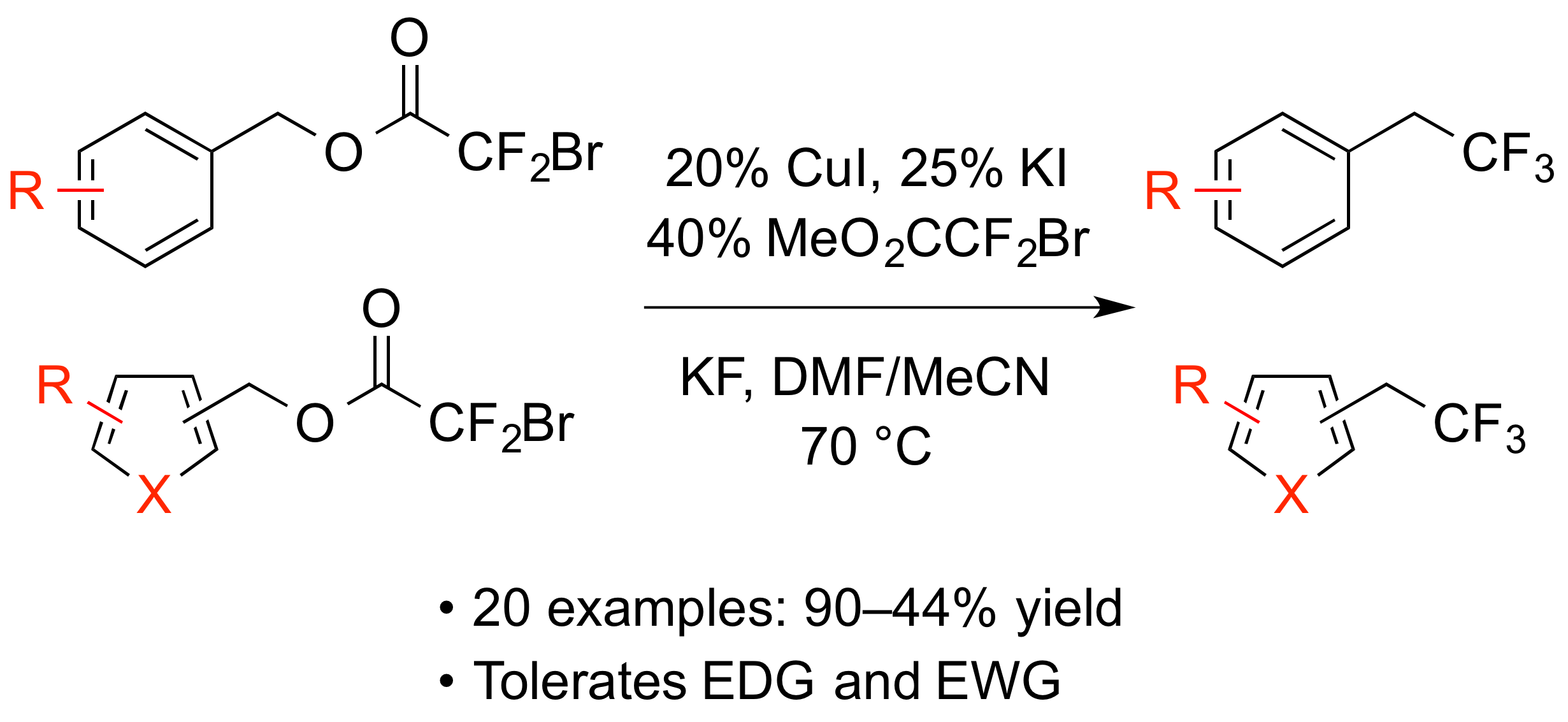
21. Copper-Catalyzed Synthesis of Trifluoroethylarenes from Benzylic Bromodifluoroacetates
Ambler, B. R.; Zhu, L.; Altman, R. A.*
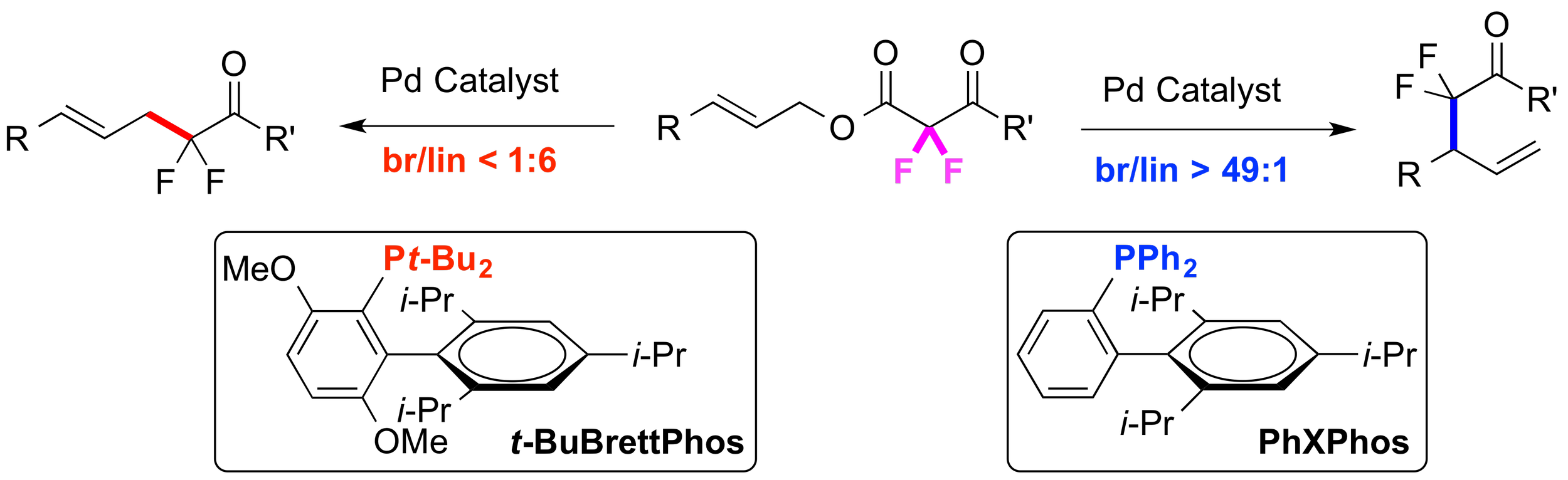
20. Ligand-controlled Regioselective Cu-Catalyzed Trifluoromethylation to Generate Trifluoromethyl Allenes
Ambler, B. R.; Peddi, S.; Altman, R. A.*
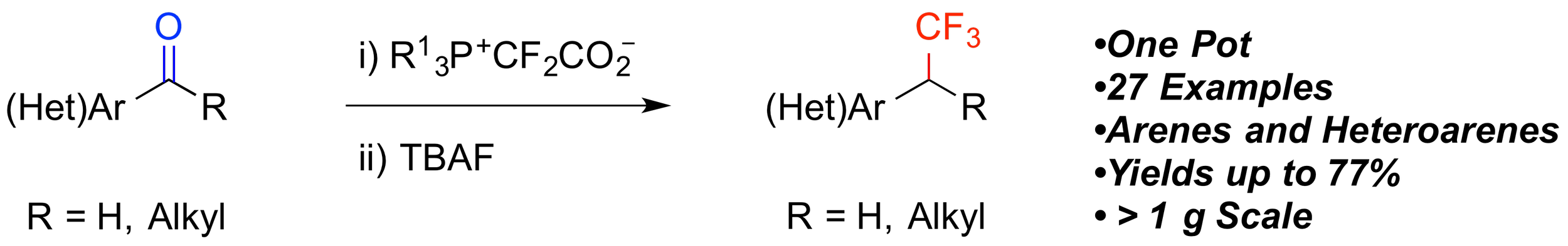
18. Metal-free Trifluoromethylation of Aromatic and Heteroaromatic Aldehydes and Ketones
Qiao, Y.; Si, T.; Yang, M.-H.; Altman, R. A.*
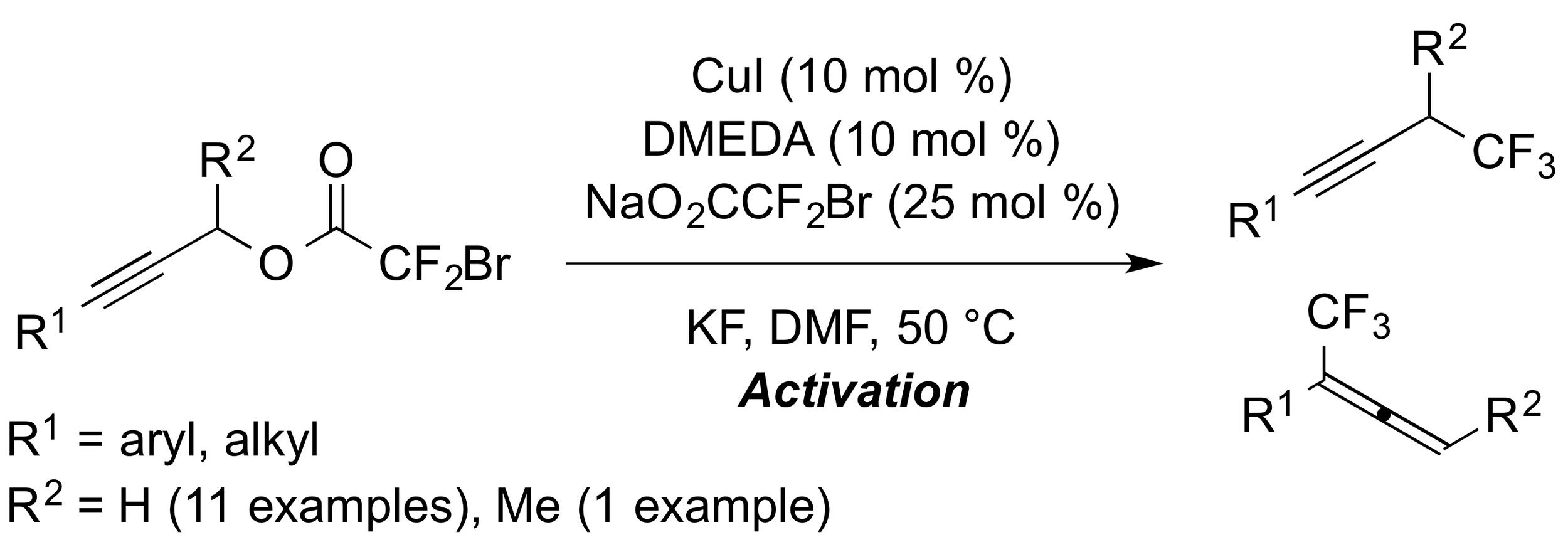
17. Copper-catalyzed Conversion of Propargylic Bromodifluoroacetates to Trifluoromethanes
Ambler, B. R.; Peddi, S.; Altman, R. A.*
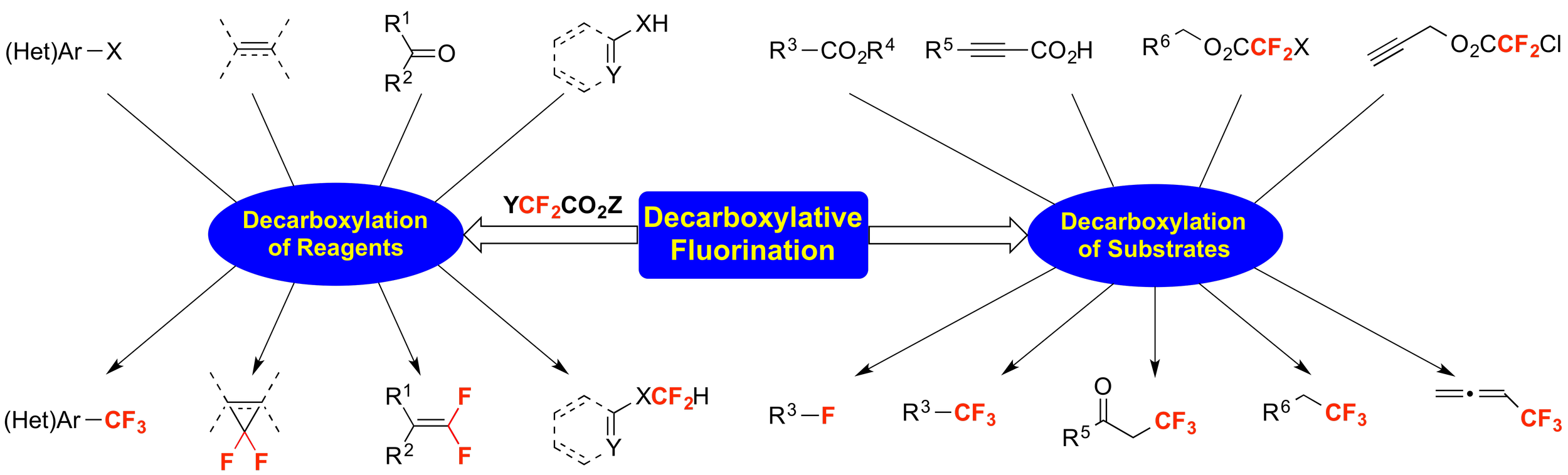
16. Decarboxylative Fluorination Strategies for Accessing Medicinally-Relevant Products
Qiao, Y.; Ambler, B. R.; Zhu, L.; Altman, R. A.*
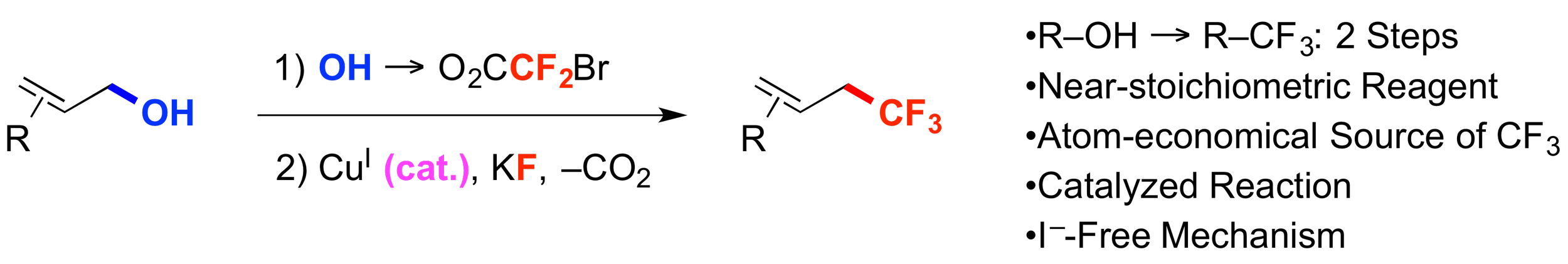
15. Copper-Catalyzed Trifluoromethylation of Allylic Bromodifluoroacetic Esters
Ambler, B. R.; Altman, R. A.*
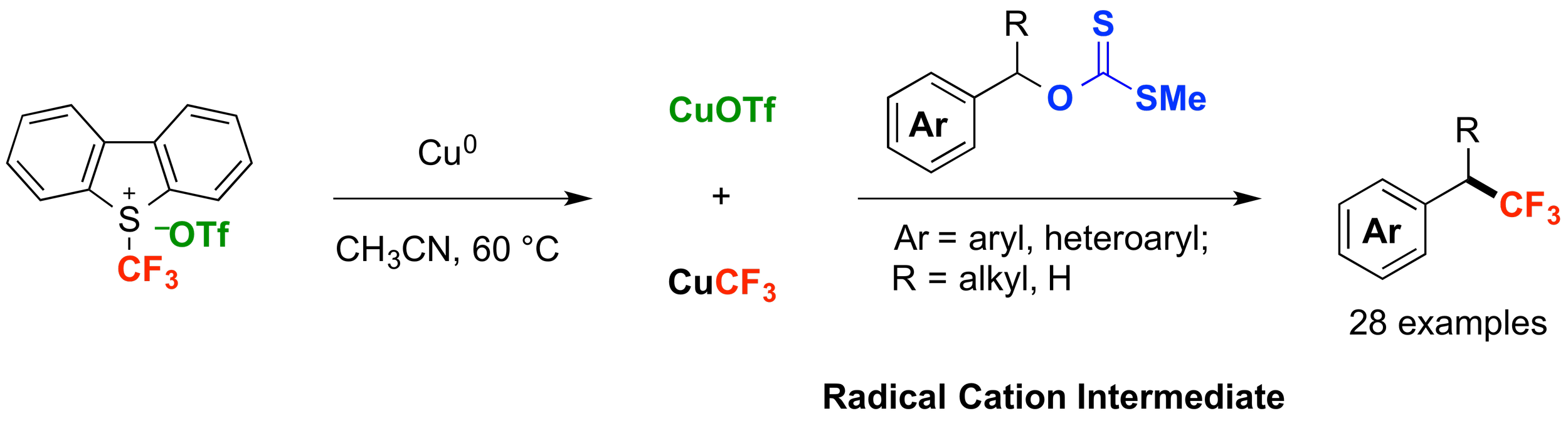
14. Copper-Mediated Deoxygenative Trifluoromethylation of Benzylic Xanthates: Formation of C(sp3)–CF3 from an O-Based Electrophile
Zhu, L.; Liu, S.; Douglas, J. T.; Altman, R. A.*