
56. Discovery of RGS2-FBXO44 interaction inhibitors using a cell-based NanoBit assay
Aryal, S.; Wong, C. S. Y.; McNabb, H. J.; Junaid, A.; Altman, R. A.; Sjögren, B.
Molecular Pharmacology 2025, https://doi.org/10.1016/j.molpha.2025.100030

55. A Deoxyfluoroalkylation-Aromatization Strategy to Access Fluoroalkyl Arenes
Bhattarai, P.; Koley, S. K.; Goel, K.; Altman, R. A.*

54. Synthesis of 4-(2,2-Difluorovinyl)benzonitrile through a Wittig-type Olefination of 4-Formylbenzonitrile
Intelli. A. J.; Sorrentino, J. P.; Altman, R. A.*

53. A Phenotypic High-Throughput Screen Identifies Small Molecule Modulators of Endogenous RGS10 in BV-2 Cells
Talele, S.; Gonzalez, S.; Trudeau, J.; Junaid, A.; Loy, C.; Altman, R. A.; Sjogren, J. B.*

52. α-Amino-β-Carboxymuconate-ε-Semialdehyde Decarboxylase Catalyzes Enol/Keto Tautomerization of Oxaloacetate
Yang, Y.; Davis, I.; Altman, R. A.; Liu, A.*

51. Palladium and Copper Co-Catalyzed Chloro-Arylation of gem-Difluorostyrenes – Use of a Nitrite Additive to Suppress β-F Elimination
Intelli, A. J.; Wayment, C. Z.; Lee, R. T.; Yuan, K.; Altman, R. A.*

50. Deoxytrifluoromethylation/Aromatization of Cyclohexan(en)ones to Access Highly Substituted Trifluoromethylarenes
Bhattarai, P.; Abd El-Gaber, M. K.; Koley, S. K.; Altman, R. A.*

49. Peroxide-Initiated Hydrophosphination of gem-Difluoroalkenes
Intelli, A. J.; Lee, R. T.; Altman, R. A.*

48. Palladium-Catalyzed Dearomatization of Benzothiophenes: Isolation and Functionalization of a Discrete Dearomatized Intermediate
Intelli, A. J.; Pal, M.; Selvaraju, M.; Altman, R. A.*

47. A Diselenide Additive Enables Photocatalytic Hydroalkoxylation of gem-Difluoroalkenes
Herrick, R. M.; Abd El-Gaber, M. K.; Rodriguez, L. G. C.; Altman, R. A.*
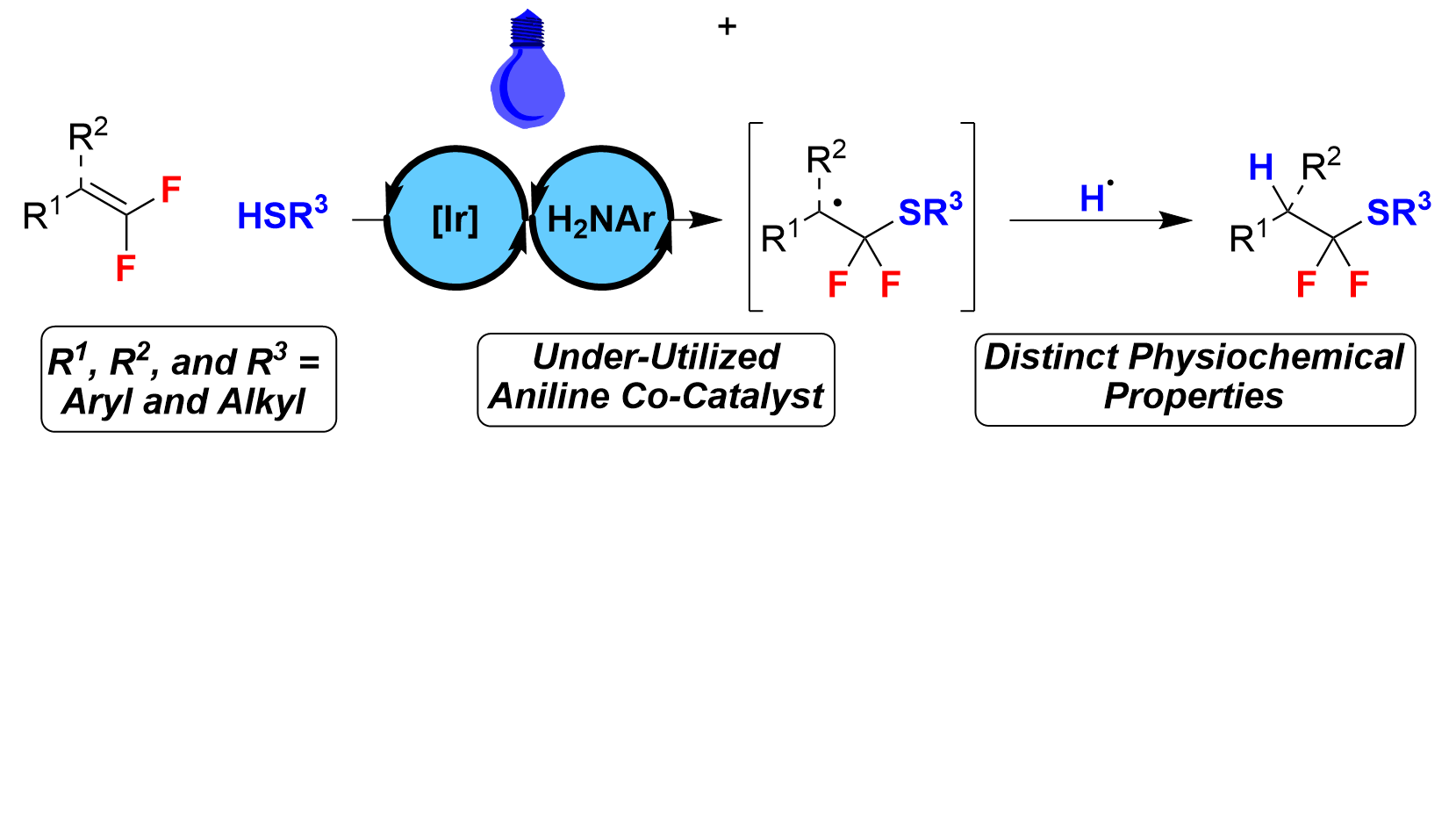
46. Photocatalytic Hydrothiolation of gem-Difluoroalkenes
Sorrentino, J. P.; Herrick, R. M.; Abd El-Gaber, M. K.; Abdelazem, A. Z.; Altman, R. A.*

45. Cu(II)-Catalyzed Oxidation of gem-Difluoroalkenes Generate α,α-Difluorinated-α-Phenoxyketones
Koley, S.; Cayton, K. T.; González-Montiel, G. A.; Yadav, M. R.; Orsi, D. L.; Intelli, A. J.; Cheong, P. H.-Y.;* Altman, R. A.*

44. Fluoroalkylation of Dextromethorphan Improves CNS Exposure and Metabolic Stability
Sorrentino, J. P.; Altman, R. A.*

43. Modulating β-Arrestin-2 Recruitment at the δ- and μ-Opioid Receptors
Sharma K. S.; Cassell, R. J.; Meqbil, Y. J.; Su, H.; Blaine, A. T.; Cummins, B. R.; Mores, K. L.; Johnson, D.; van Rijn, R. M.;* Altman, R. A.*
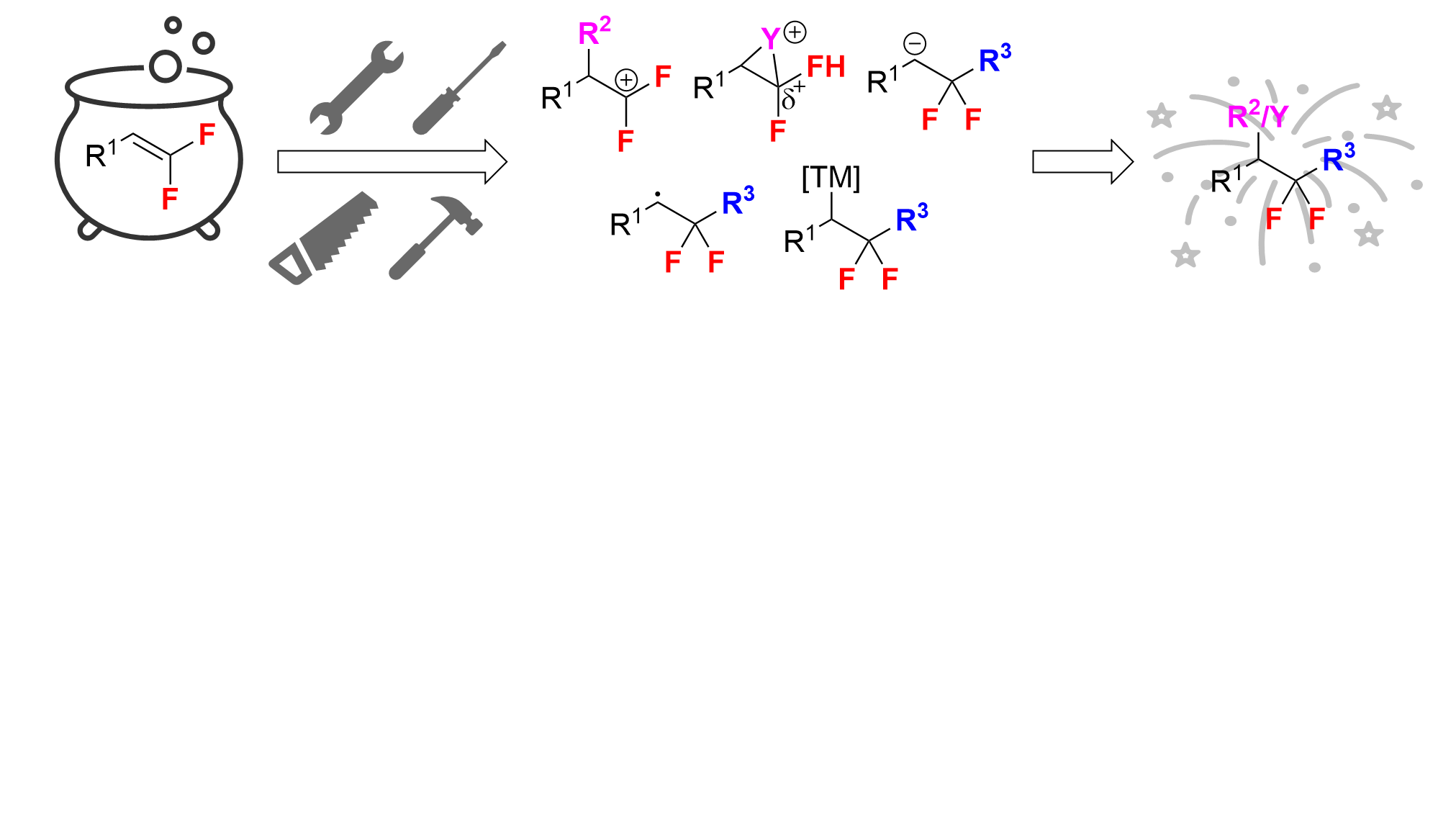
42. Fluorine-Retentive Strategies for the Functionalization of gem-Difluoroalkenes
Sorrentino, J. P.; Altman, R. A.*

41. Acid-catalyzed Hydrothiolation of gem-Difluorostyrenes to Access α,α-Difluoroalkylthioethers
Sorrentino, J. P.; Orsi, D. L.; Altman, R. A.*
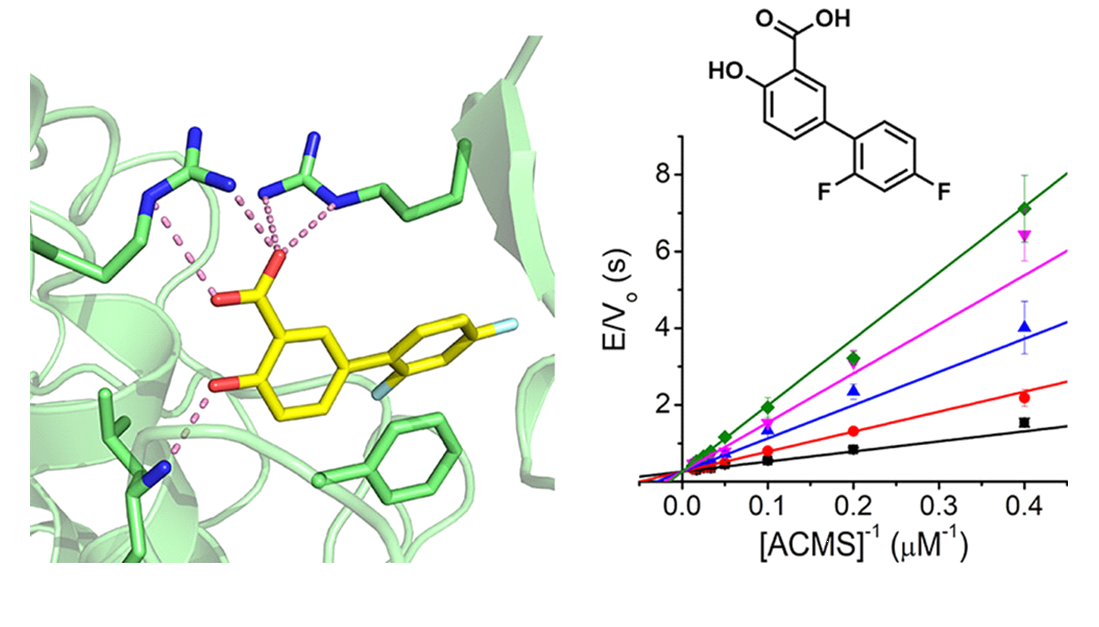
40. Diflunisal Derivatives as Modulators of ACMS Decarboxylase Targeting the Tryptophan-Kynurenine Pathway
Yang, Y.; Borel, T.; de Azambuja, F.; Johnson, D.; Sorrentino, J. P.; Udokwu, C.; Davis, I.; Liu, A.;* Altman, R. A.*

39. Arylation of gem-difluoroalkenes using a Pd/Cu Co-catalytic system that avoids β-fluoride elimination
Yuan, K.; Feoktistova, T.; Cheong, P. H.-Y.;* Altman, R. A.*

38. Cobalt-Catalyzed Selective Unsymmetric Dioxidation of β,β-Difluorostyrenes
Orsi, D. L.; Sorrentino, J. P.; Douglas, J. T.; Altman, R. A.*

37. Late-Stage Conversion of a Metabolically Labile Aryl Methyl Ether-Containing Natural Product to Fluoroalkyl Analogues
Sorrentino, J. S.; Ambler, B. R.; Altman, R. A.*