
54. Synthesis of 4-(2,2-Difluorovinyl)benzonitrile through a Wittig-type Olefination of 4-Formylbenzonitrile
Intelli. A. J.; Sorrentino, J. P.; Altman, R. A.*

51. Palladium and Copper Co-Catalyzed Chloro-Arylation of gem-Difluorostyrenes – Use of a Nitrite Additive to Suppress β-F Elimination
Intelli, A. J.; Wayment, C. Z.; Lee, R. T.; Yuan, K.; Altman, R. A.*

49. Peroxide-Initiated Hydrophosphination of gem-Difluoroalkenes
Intelli, A. J.; Lee, R. T.; Altman, R. A.*

47. A Diselenide Additive Enables Photocatalytic Hydroalkoxylation of gem-Difluoroalkenes
Herrick, R. M.; Abd El-Gaber, M. K.; Rodriguez, L. G. C.; Altman, R. A.*
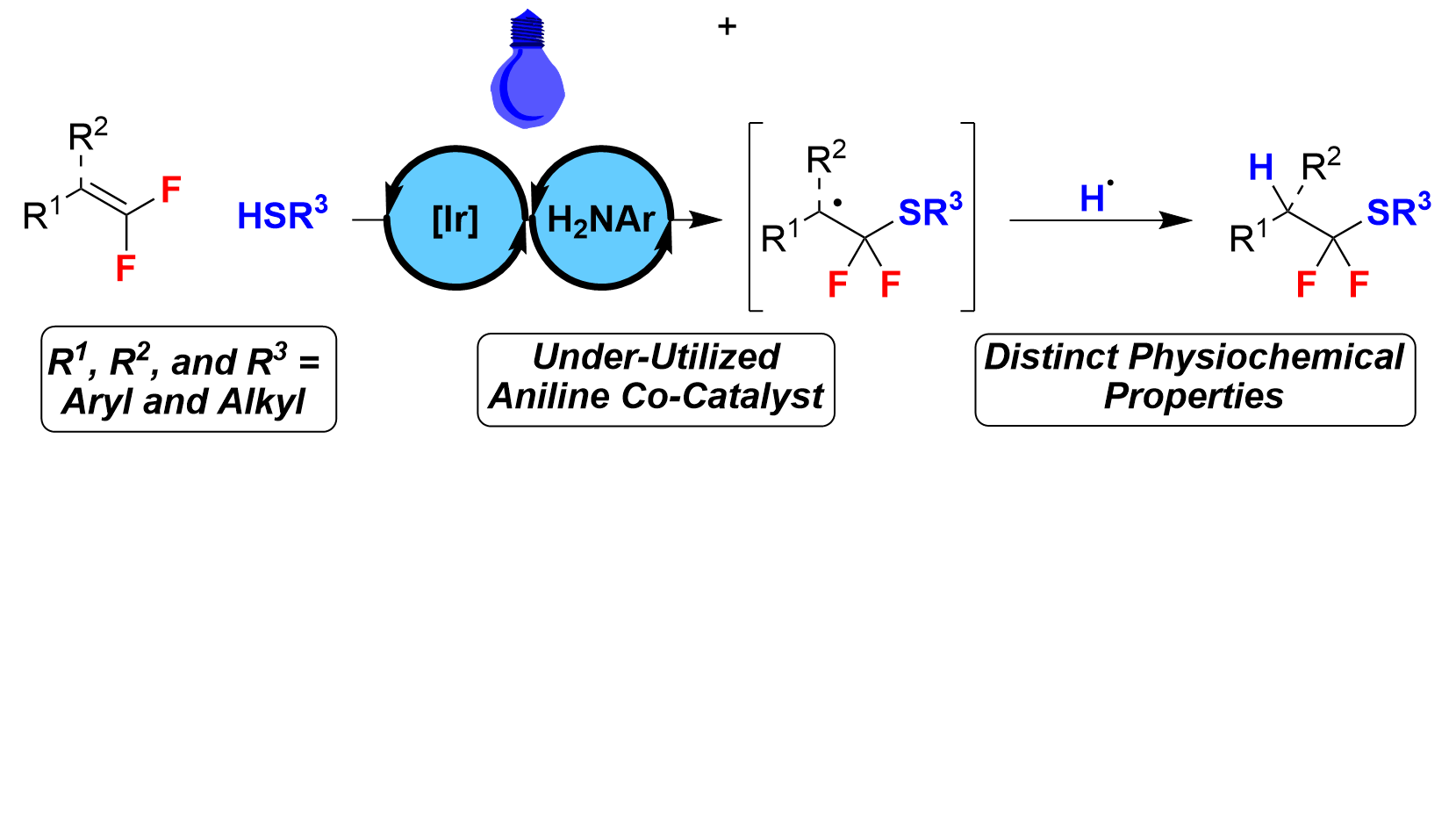
46. Photocatalytic Hydrothiolation of gem-Difluoroalkenes
Sorrentino, J. P.; Herrick, R. M.; Abd El-Gaber, M. K.; Abdelazem, A. Z.; Altman, R. A.*

45. Cu(II)-Catalyzed Oxidation of gem-Difluoroalkenes Generate α,α-Difluorinated-α-Phenoxyketones
Koley, S.; Cayton, K. T.; González-Montiel, G. A.; Yadav, M. R.; Orsi, D. L.; Intelli, A. J.; Cheong, P. H.-Y.;* Altman, R. A.*
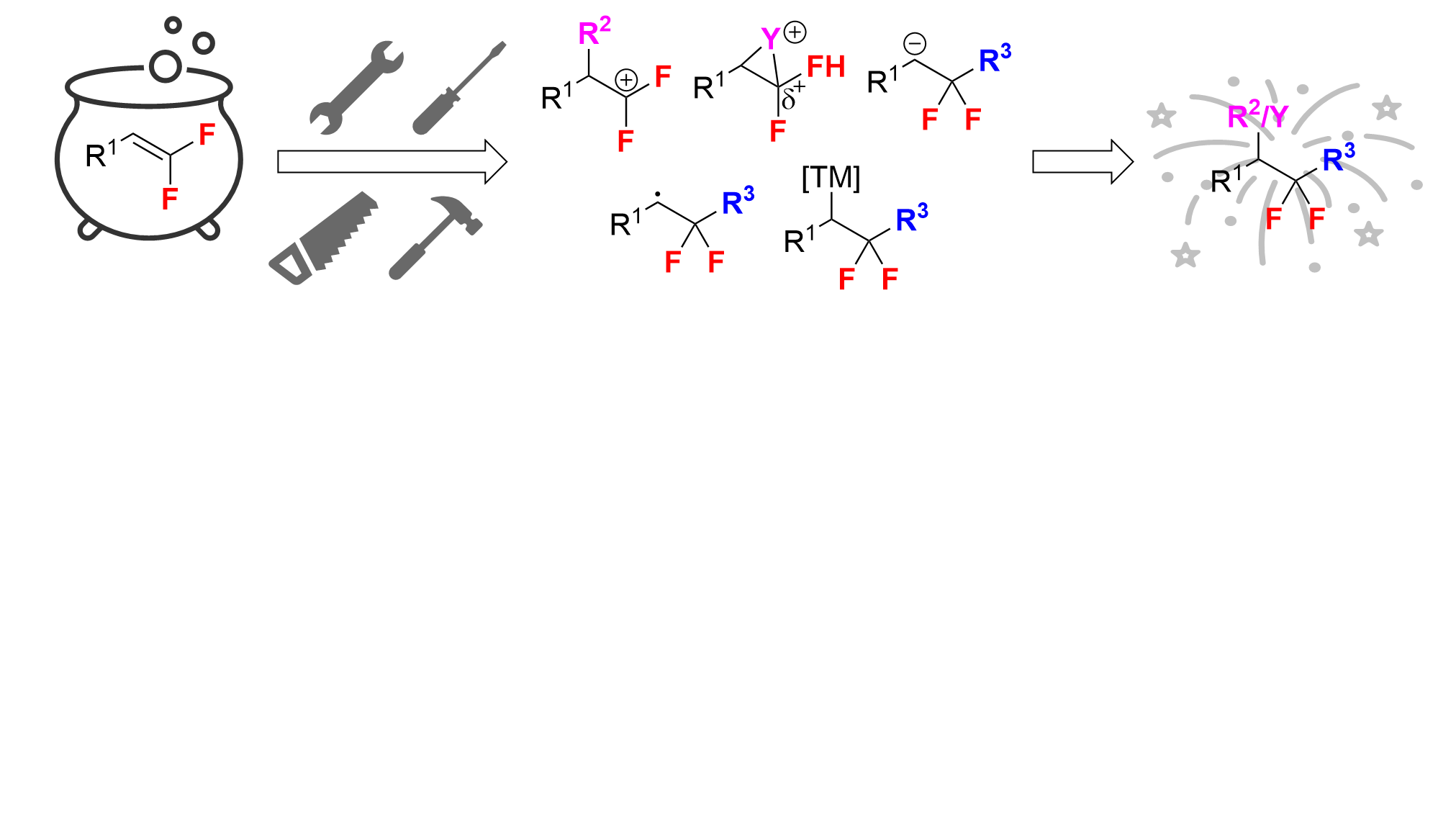
42. Fluorine-Retentive Strategies for the Functionalization of gem-Difluoroalkenes
Sorrentino, J. P.; Altman, R. A.*

41. Acid-catalyzed Hydrothiolation of gem-Difluorostyrenes to Access α,α-Difluoroalkylthioethers
Sorrentino, J. P.; Orsi, D. L.; Altman, R. A.*

39. Arylation of gem-difluoroalkenes using a Pd/Cu Co-catalytic system that avoids β-fluoride elimination
Yuan, K.; Feoktistova, T.; Cheong, P. H.-Y.;* Altman, R. A.*

38. Cobalt-Catalyzed Selective Unsymmetric Dioxidation of β,β-Difluorostyrenes
Orsi, D. L.; Sorrentino, J. P.; Douglas, J. T.; Altman, R. A.*

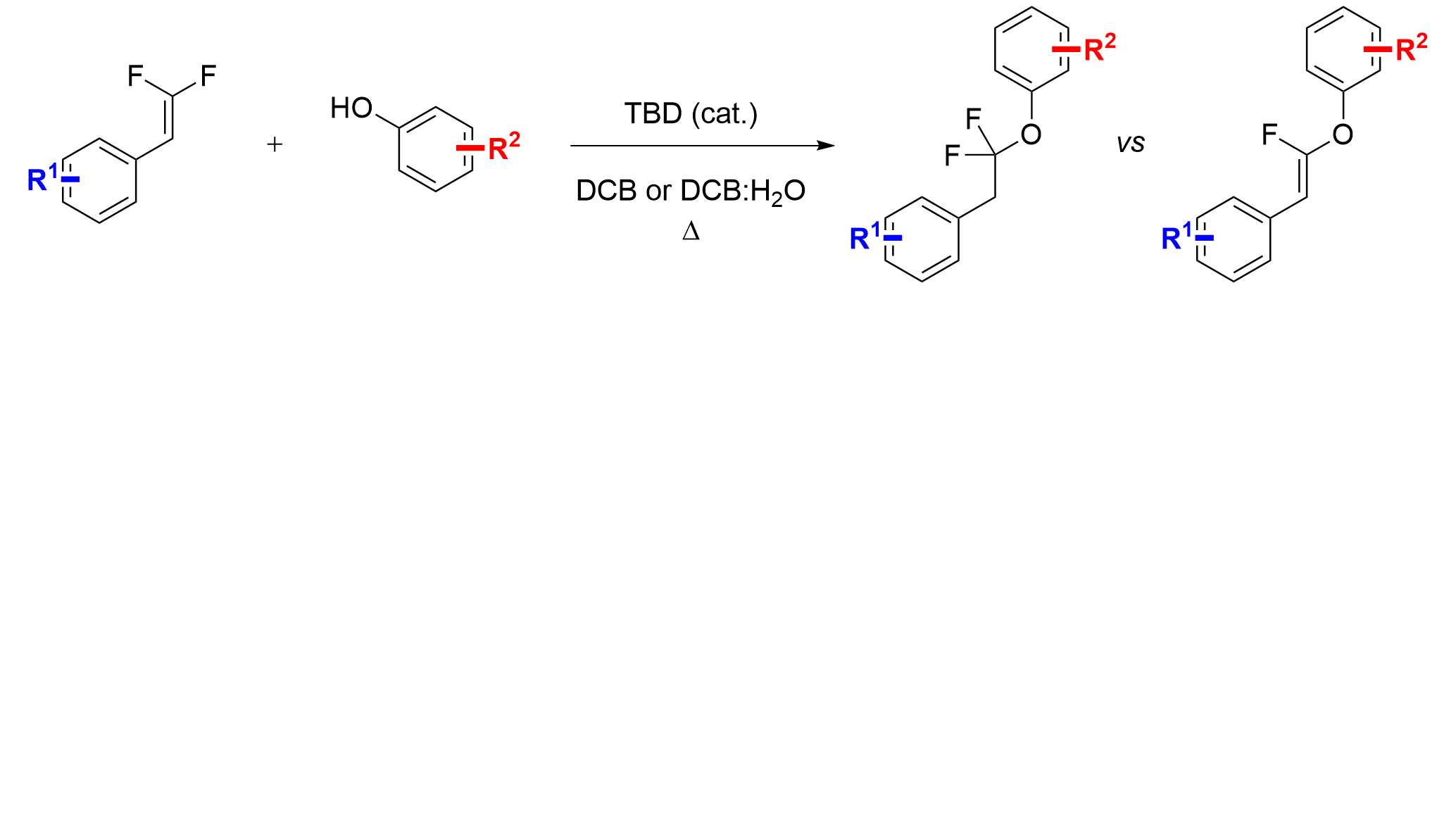
32. Organocatalytic Strategy for Hydrophenolation of gem-Difluoroalkenes
Orsi, D. L.; Yadav, M. R.; Altman, R. A.*
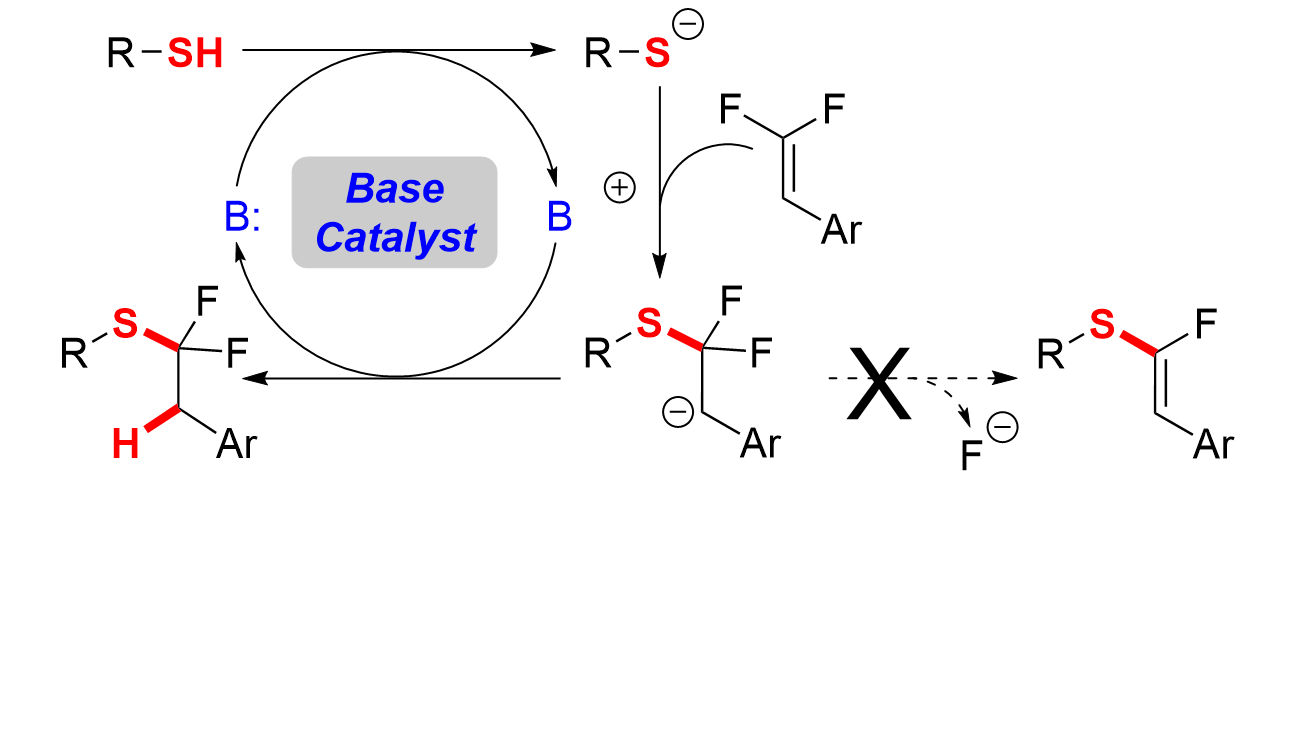
25. Base Catalysis Enables Access to α,α-Difluoroalkylthio-ethers
Orsi, D. L.; Easley, B. J.; Lick, A. M.; Altman, R. A.*

18. Metal-free Trifluoromethylation of Aromatic and Heteroaromatic Aldehydes and Ketones
Qiao, Y.; Si, T.; Yang, M.-H.; Altman, R. A.*

13. Preparation of Fluoroalkenes via the Shapiro Reaction: Direct Access to Fluorinated Peptidomimetics
Yang, M. H.; Matikonda, S. S.; Altman, R. A.*