
59. Insights into Deoxytrifluoromethylation /Aromatization to Access Highly Substituted Trifluoromethyl Arenes
Bhattarai, P.; Abd El-Gaber, M. K.; Koley, S.K.; Altman, R. A.*

58. Unified Enantioselective Synthesis of 5-Phenylmorphans and cis-Octahydroisoquinolines
Mahgoub, A.; Khamrai, J.; Festus, O.; Altman, R. A.*

57. NBS-Promoted Synthesis of Maleimides by Oxidative Dearomatization of Pyrroles: Mechanistic Investigations Using Online MS in Real-Time
Kumar, A.; Mishra, P. K.; Nandy, A.; Altman, R. A.; Banerjee, S.; Verma, A. K.*
Org. Lett. 2025, 27, 7810–7815.
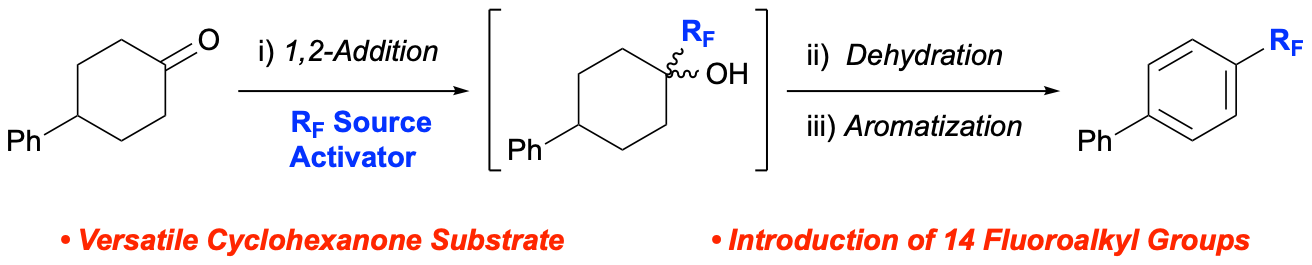
55. A Deoxyfluoroalkylation-Aromatization Strategy to Access Fluoroalkyl Arenes
Bhattarai, P.; Koley, S. K.; Goel, K.; Altman, R. A.*
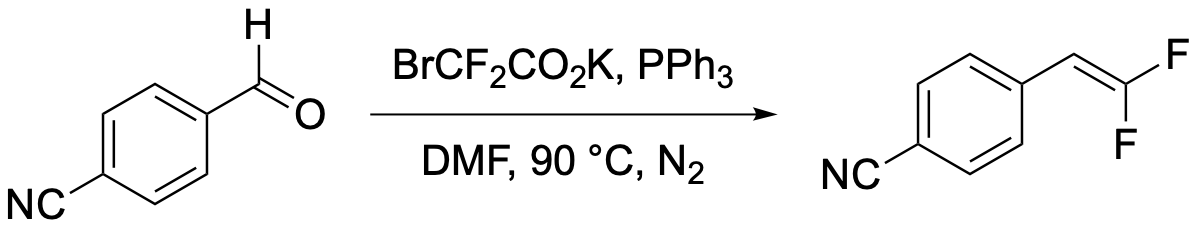
54. Synthesis of 4-(2,2-Difluorovinyl)benzonitrile through a Wittig-type Olefination of 4-Formylbenzonitrile
Intelli. A. J.; Sorrentino, J. P.; Altman, R. A.*
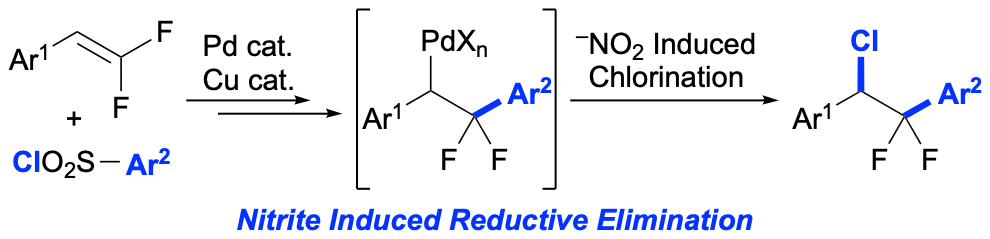
51. Palladium and Copper Co-Catalyzed Chloro-Arylation of gem-Difluorostyrenes – Use of a Nitrite Additive to Suppress β-F Elimination
Intelli, A. J.; Wayment, C. Z.; Lee, R. T.; Yuan, K.; Altman, R. A.*
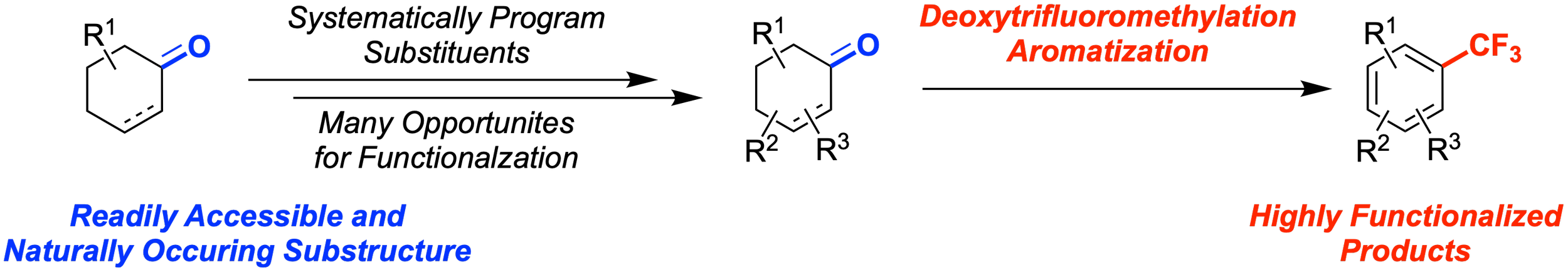
50. Deoxytrifluoromethylation/Aromatization of Cyclohexan(en)ones to Access Highly Substituted Trifluoromethylarenes
Bhattarai, P.; Abd El-Gaber, M. K.; Koley, S. K.; Altman, R. A.*

49. Peroxide-Initiated Hydrophosphination of gem-Difluoroalkenes
Intelli, A. J.; Lee, R. T.; Altman, R. A.*
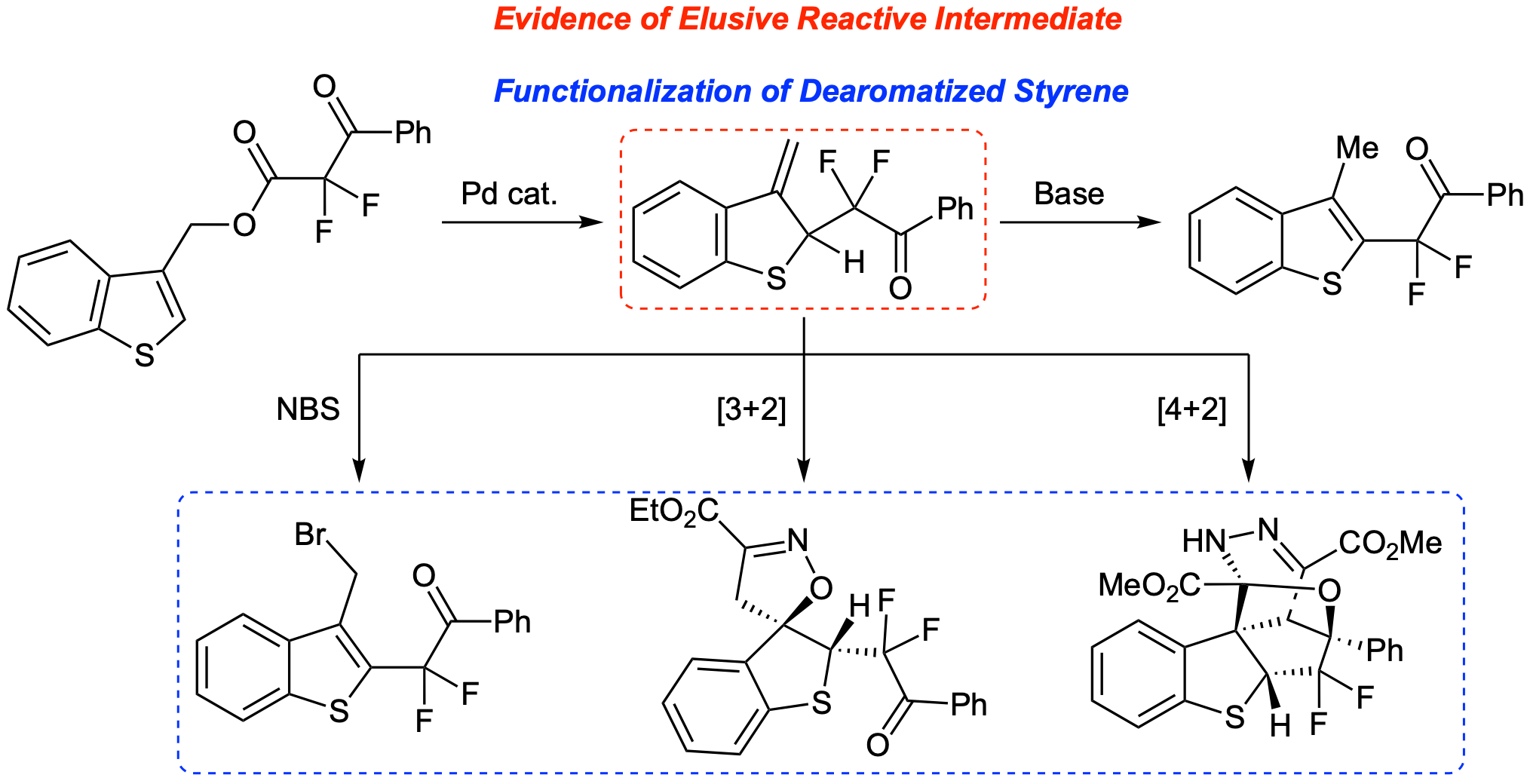
48. Palladium-Catalyzed Dearomatization of Benzothiophenes: Isolation and Functionalization of a Discrete Dearomatized Intermediate
Intelli, A. J.; Pal, M.; Selvaraju, M.; Altman, R. A.*
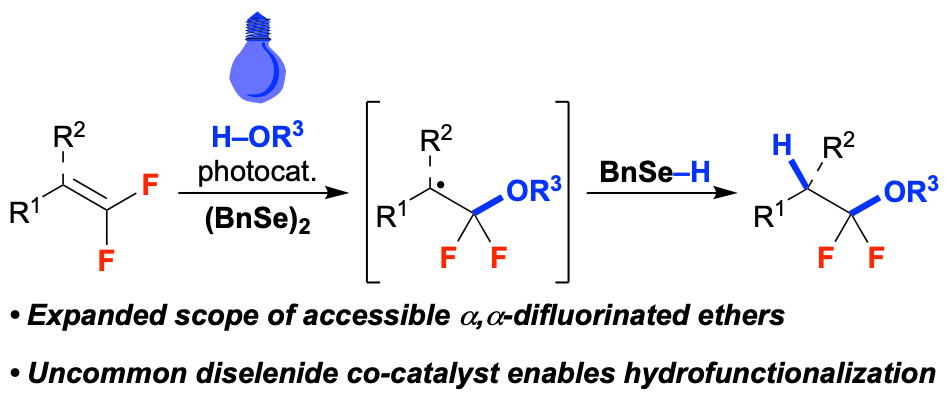
47. A Diselenide Additive Enables Photocatalytic Hydroalkoxylation of gem-Difluoroalkenes
Herrick, R. M.; Abd El-Gaber, M. K.; Rodriguez, L. G. C.; Altman, R. A.*
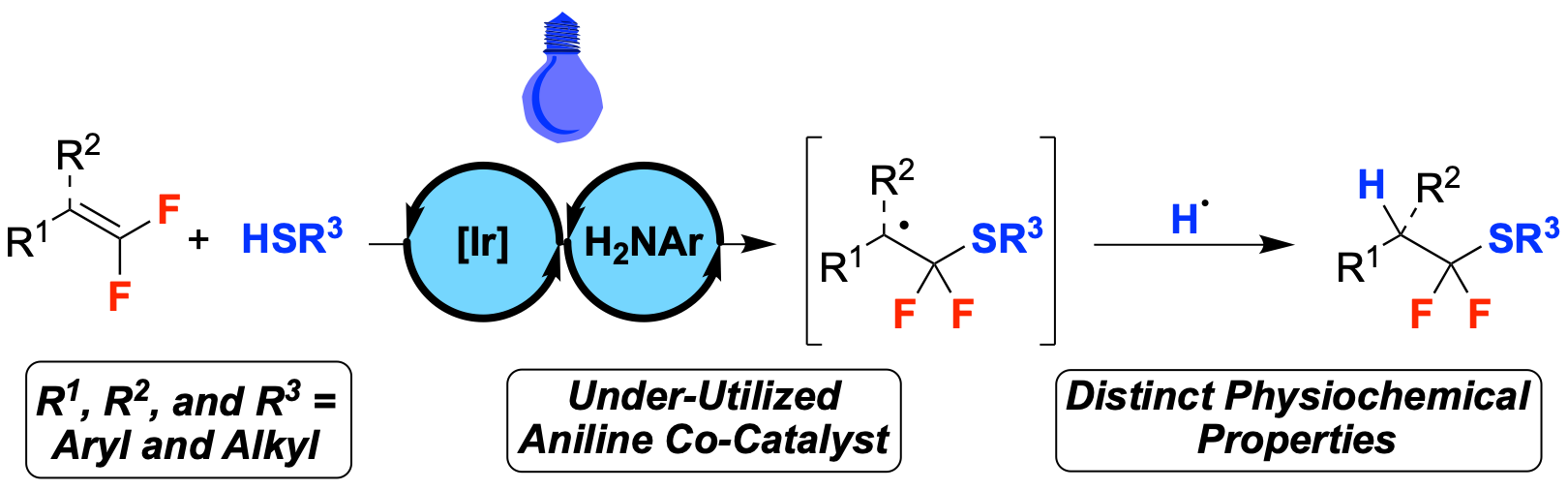
46. Photocatalytic Hydrothiolation of gem-Difluoroalkenes
Sorrentino, J. P.; Herrick, R. M.; Abd El-Gaber, M. K.; Abdelazem, A. Z.; Altman, R. A.*
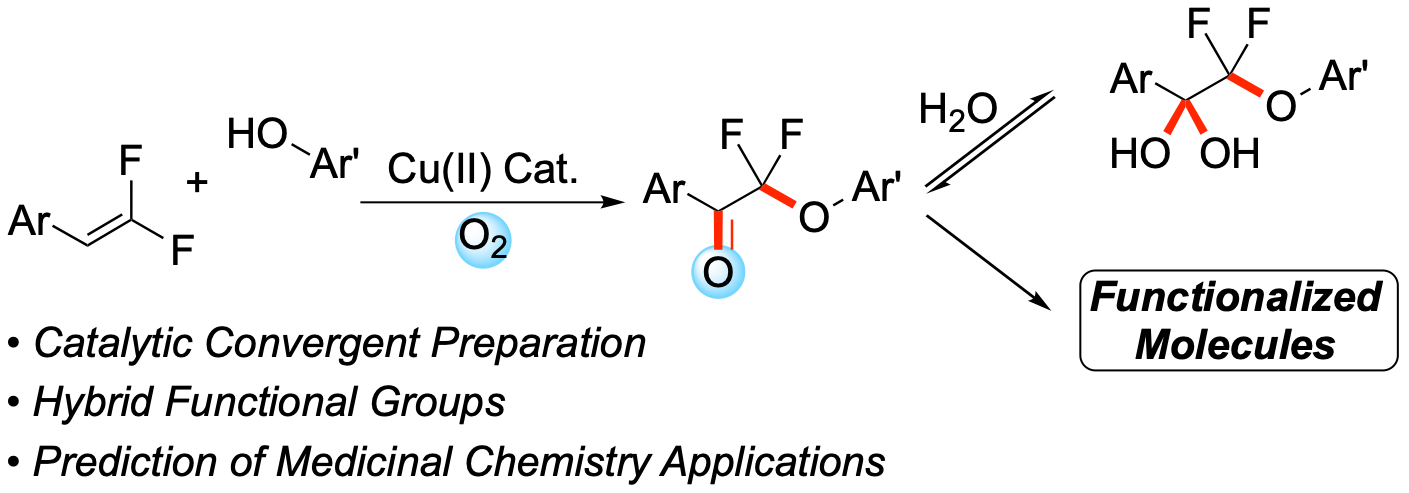
45. Cu(II)-Catalyzed Oxidation of gem-Difluoroalkenes Generate α,α-Difluorinated-α-Phenoxyketones
Koley, S.; Cayton, K. T.; González-Montiel, G. A.; Yadav, M. R.; Orsi, D. L.; Intelli, A. J.; Cheong, P. H.-Y.;* Altman, R. A.*
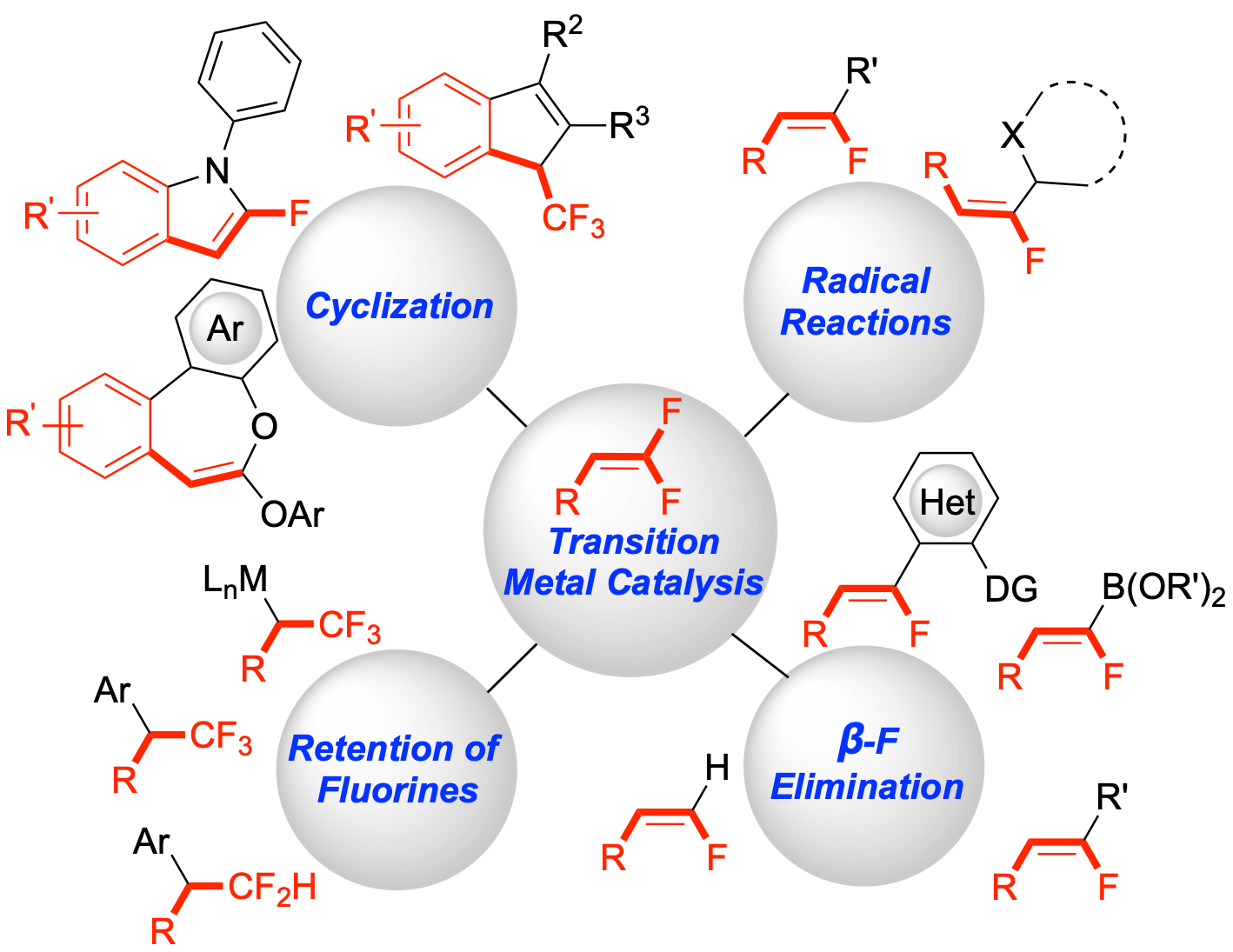
42. Fluorine-Retentive Strategies for the Functionalization of gem-Difluoroalkenes
Sorrentino, J. P.; Altman, R. A.*
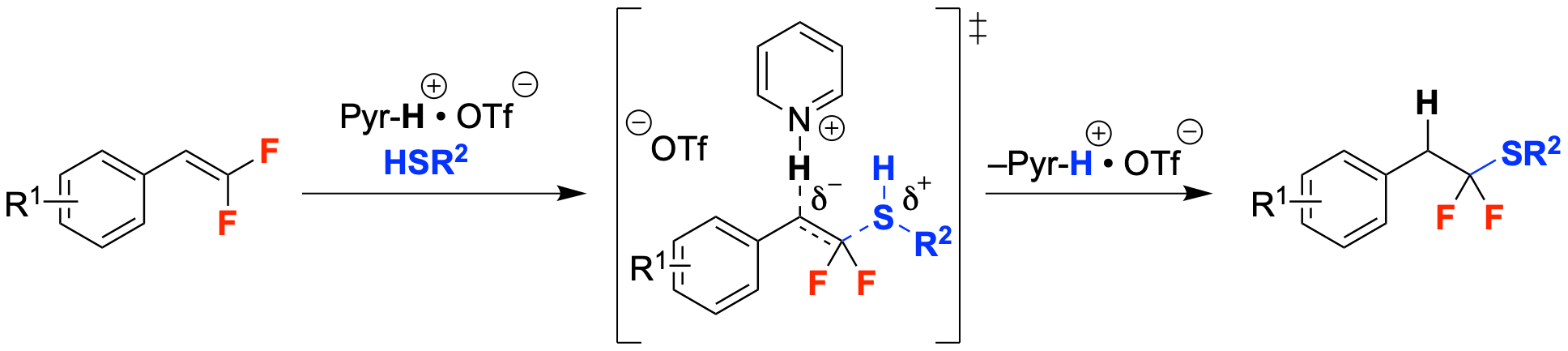
41. Acid-catalyzed Hydrothiolation of gem-Difluorostyrenes to Access α,α-Difluoroalkylthioethers
Sorrentino, J. P.; Orsi, D. L.; Altman, R. A.*
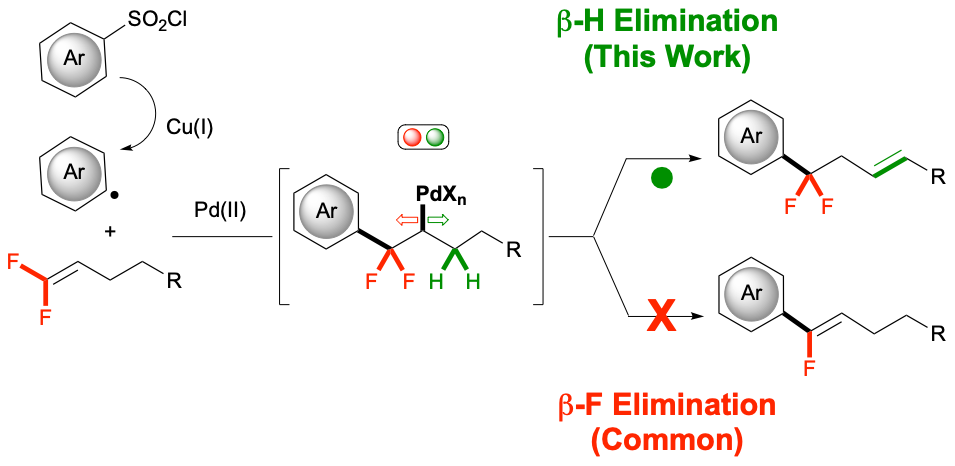
39. Arylation of gem-difluoroalkenes using a Pd/Cu Co-catalytic system that avoids β-fluoride elimination
Yuan, K.; Feoktistova, T.; Cheong, P. H.-Y.;* Altman, R. A.*
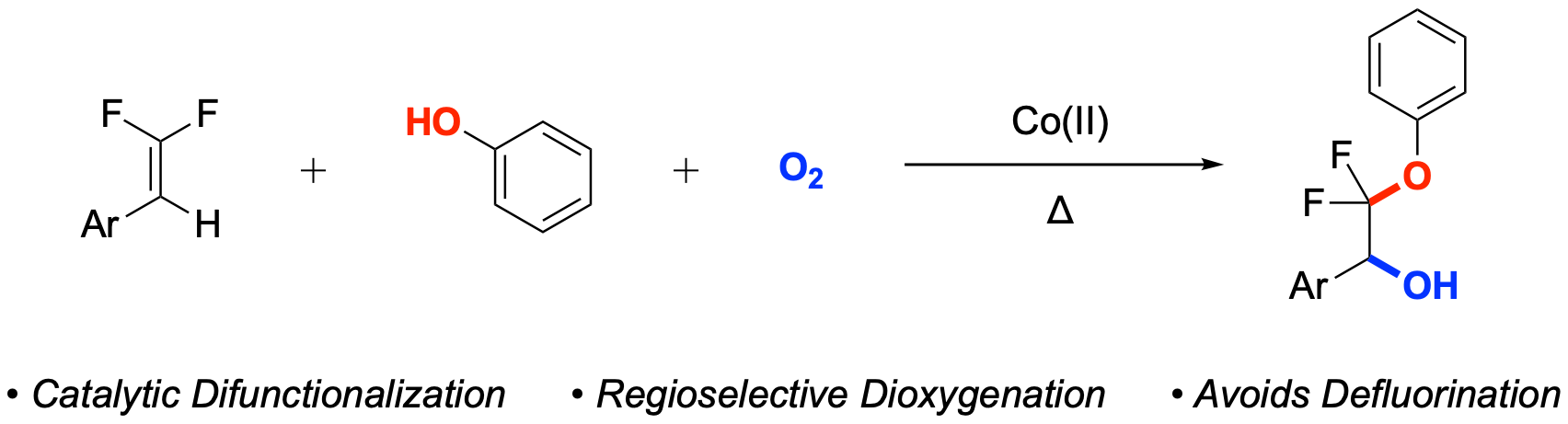
38. Cobalt-Catalyzed Selective Unsymmetric Dioxidation of β,β-Difluorostyrenes
Orsi, D. L.; Sorrentino, J. P.; Douglas, J. T.; Altman, R. A.*
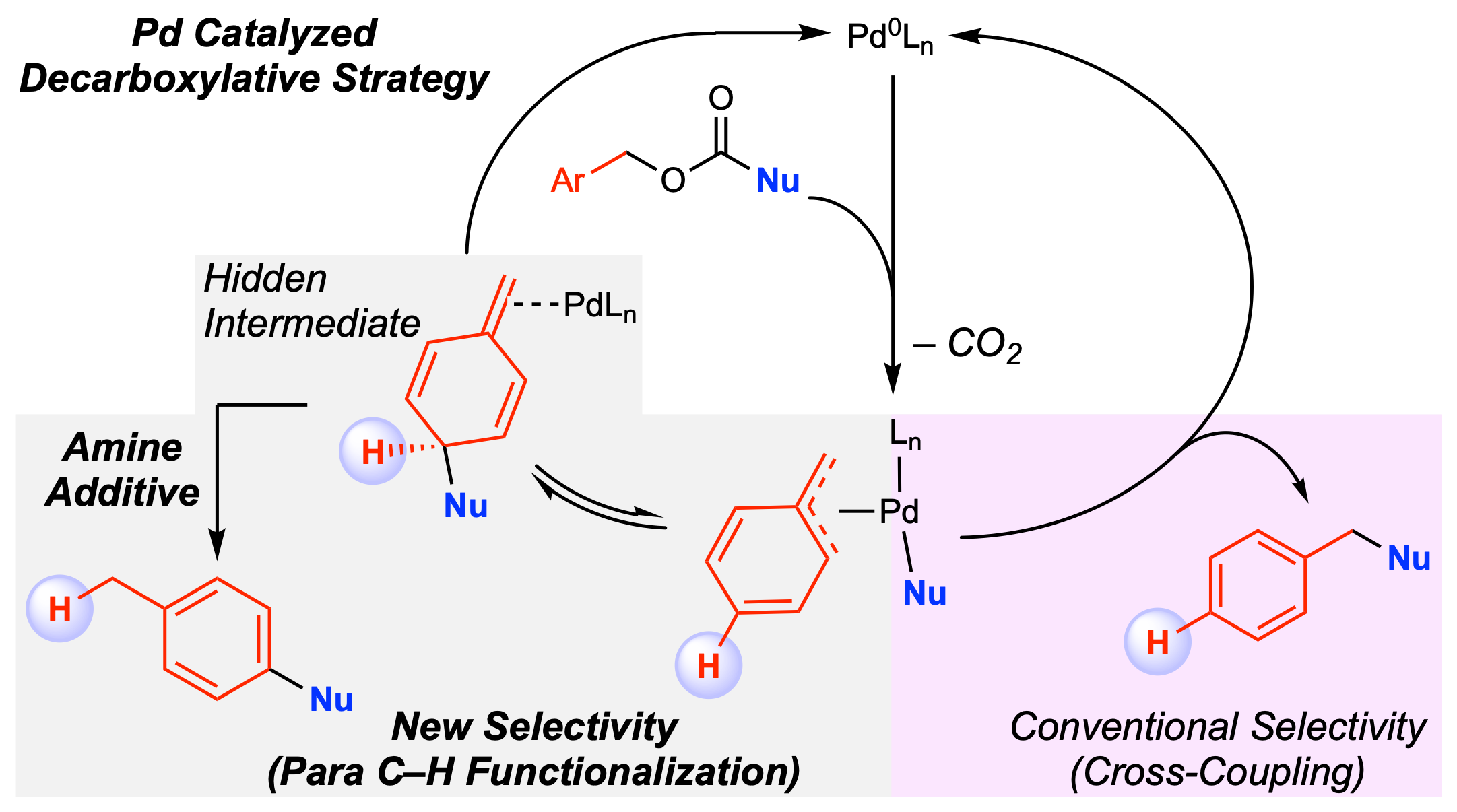
35. Connecting Remote C–H Bond Functionalization and Decarboxylative Coupling Using Simple Amines
de Azambuja, F.; Yang, M.-H.; Feoktistova, T.; Selvaraju, M.; Brueckner, A. C.; Grove, M. A.; Koley, S.; Cheong, P. H.-Y.;* Altman, R. A.*
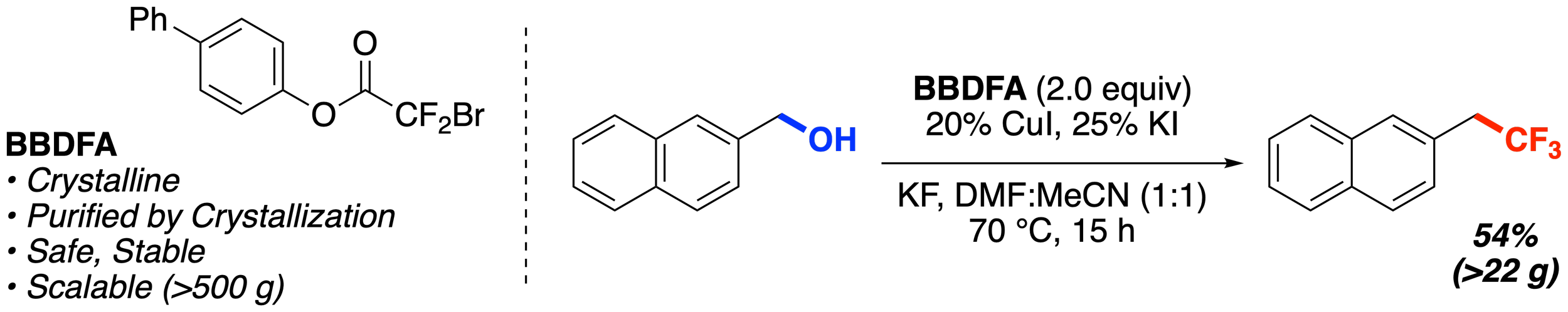
33. BBDFA: A Practical Reagent for Trifluoromethylation of Allylic and Benzylic Alcohols on Preparative Scale
Han, C.;* Alabanza, L. M.; Kelly, S. M.; Orsi, D. L.; Gosselin, F.; Altman, R. A.*

32. Organocatalytic Strategy for Hydrophenolation of gem-Difluoroalkenes
Orsi, D. L.; Yadav, M. R.; Altman, R. A.*